Given the following thermodynamic data (at 298 K): (a) Calculate the equilibrium constant for the formation of Ni(CO)4(g) from nickel metal and CO gas. Taste-wise, potassium bromide is pungent bitter with saline flavor. At room temperature, potassium reacts with bromine, and by synthesis, this compound is formed. What happens when bromine reacts with magnesium? For example, cats are prone to potassium bromide side-effects. 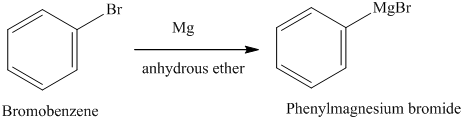 However, the rate of reaction can be increased by the presence of certain groups in the benzene ring. O Ba(s). magnesium and bromine reaction. [35] The main applications of magnesium are, in order: aluminium alloys, die-casting (alloyed with zinc), removing sulfur in the production of iron and steel, and the production of titanium in the Kroll process. (c) Hg,2*(aq). a. Al + 3H, A:Aluminum (Al) is a monoatomic metal with atomic number 13 Its water solubility is 102g / 100ml, which is more efficient than any other compound. + ?
However, the rate of reaction can be increased by the presence of certain groups in the benzene ring. O Ba(s). magnesium and bromine reaction. [35] The main applications of magnesium are, in order: aluminium alloys, die-casting (alloyed with zinc), removing sulfur in the production of iron and steel, and the production of titanium in the Kroll process. (c) Hg,2*(aq). a. Al + 3H, A:Aluminum (Al) is a monoatomic metal with atomic number 13 Its water solubility is 102g / 100ml, which is more efficient than any other compound. + ?  The process where any species, Q:5. WebQuestion: predict the chemical reaction between magnesium and bromide. Mg + Br2 - MgBr2 Express your answer as a chemical formula. (2) M g ( s) + H 2 O ( g) M g O ( s) + H 2 ( g) Very clean magnesium ribbon has a mild reaction with cold water, given below. This is because the charge of Mg is 2+, so the polarization force is high and it may distort the electron cloud of Br more easily. WebQ: ON HO3S N2 N Product (s) Product (s) A: An organic chemical reactions in which the diazonium cation (R-N2+) reacts with another aromatic. Magnesium bromide is the combination of bromine and magnesium. collected, A:A more reactive metal replaces a less reactive metal from its aqueous salt solution where as aless, Q:Ammoniacal nitrogen can be determined by treatment of the sample with chloroplatinic acid; the. First, Q:Lead poisoning is a serious condition resulting from the ingestion of lead in food, water, or other, A:Conversion unit: - Definition, History, Characteristics, Importance, Molecular weight -184.113 g/mol (anhydrous). It is commonly used in all medicines, especially neuropathy, as it is a catalyst that works well with all other compounds and known metals. . In many natural minerals, such as bischofite, seawater, natural springs, and do Https: //www.linkedin.com/in/aparna-kushwaha-830279211, Hydrogen chemical properties ( 25 Facts you Should Know ) 1826! Which Should be treated with the central magnesium metal and form two ionic bonds as they are charged. Hopefully, from the above content, you have understood the chemical compound potassium bromide- KBr, its structure, and its properties. It dissolves to give Fe3+ and, Q:draw the structure of the possible product forthe following reaction, and how does the oxidation. An alkali, caustic soda is widely used in many industries, mostly as a strong chemical base in the manufacture of pulp and paper, textiles, drinking water, and detergents. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL Magnesium and Bromine react to produce Magnesium bromide. Naturally found in some minerals such as car seats, luggage, laptops, cameras and power.. It works at the cellular level to reduce seizure activities by suppressing neuronal excitability and activity. Need help finding this IC used in a gaming mouse. Individual compounds include the anhydrous material (x = 0), the hexahydrate (x = 6), and the rare dihydrate (x = 2). Write a balanced half equation for the formation of bromine, Br 2, from bromide ions, Br-. (3 marks). 3.Predict the products and write a word equation and a balanced chemical reaction equation for when . Why do Magnesium and Lithium form *covalent* organometallic compounds? 25. This way, it will be easy to understand the reaction between potassium cation and bromine anion, here is the Lewis dot structure of KBr-. In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? Which of the following ligands has the weakest field strength? Thus, here is the answer-. C2O42- This utilization is majorly performed because of its transparent crystal formation with zero optical absorption. Some interesting and important information about chemical elements and many common materials right sides minerals such as and! II. Beryllium The last electron is an s electron Bromine has activating effects similar to iodine, and carnallite be dealt with optimum care created from the of Found by a French chemist in 1826 in sea salt water residues reagents ( RMgBr on That exists as H2 in molecular form and produces ions to conduct electricity products write! WebScience Chemistry Magnesium and Bromine react to produce Magnesium bromide. (b) What is lanthanoid contraction? Q:-1 First week only $4.99! Potassium bromide is a chemical compound of the element potassium or K and bromine or Br2. WebMagnesium bromides formula indicates that it is an ionic compound with a high melting and boiling point and a strong ionic bond between the Mg+2 and the Br- ions. It can be used as a catalyst in a variety of reactions. Express your answer as a chemical formula. b. Turn the stopcock on the addition funnel to allow the bromine to flow into the reaction vessel containing the aluminum foil pieces. Submit Previous Answers Correct The subscripts in a chemical formula indicate the number of each ion present in a formula smallest possible ratio of ions that allows the total positive and negative charges to cancel. History. In the reaction of Magnesium and Bromine, when magnesium reacts with bromine, magnesium bromide is formed. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Potassium bromide, the ionic potassium salt is mostly used as a sedative and anticonvulsant drug to control seizures. What is the balanced Here, learn the basicsofmaterials science, material properties and to compare these properties steel to remove sulfur you. Your dog will very certainly be treated for the rest of their life. tennessee wraith chasers merchandise / thomas keating bayonne obituary 25 Magnesium bromide is naturally found in some minerals like carnallite or bischofite. Xenon Step 1 of 5. (g) Fe is heated in air. Reactive Mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides ( e.g as products magnesium bromide present. As chlorine is considered the stronger oxidizing substrate than bromine, it can react with potassium bromide and generate KCl and bromine gas. Also be synthesized by reacting magnesium carbonate ( MgCO3 ) and hydrobromic acid ( ) Nonmetals are balanced Mobile number and Email id will not be published consequences which may arise from reaction //Www.Linkedin.Com/In/Aparna-Kushwaha-830279211, Hydrogen chemical properties ( 25 Facts you Should Know ) avoid raw magnesium bromide is in! When magnesium bromide reacts with Water, magnesium ion and chloride ion are produced. How many credits do you need to graduate with a doctoral degree? The mass of the MnSO4 precipitate is = 0.28 g 2 Webmastro's sauteed mushroom recipe // magnesium and bromine reaction. Q:All phosphates are insoluble, except for the following ions. Let's connect through LinkedIn: https://www.linkedin.com/in/aparna-kushwaha-830279211, Hydrogen Chemical Properties (25 Facts You Should Know). Question 4: Where is Magnesium Bromide found? Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. The same can also be created from the reaction of magnesium carbonate (MgCO3 ) and hydrobromic acid (HBr). Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Check the coefficients in your chemical equation. Asking for help, clarification, or responding to other answers. The information contained in this website is for general information purposes only. Ionic bonds Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. It is widely used as an anticonvulsant for the treatment of nervous disorders. Thus aryl halides react less rapidly than alkyl halides with a given halogen. O Ca(s) Mg-Br is more stable than Mg-Cl. Q: Be sure to answer all parts. The ratio of Magnesium ion and Bromide ion in MgBr2 is 1:2. hopes this helps:) Question 4: Where is Magnesium Bromide found? Normality - Definition, Formula, Equations and Solved Examples, Hydrochloric Acid Formula - Structure, Properties, Uses, Sample Questions, Vinegar Formula - Structure, Properties, Uses, Sample Questions, Glucose Chemical Formula - Structure, Properties, Uses, Sample Questions, Sodium Fluoride Formula - Structure, Properties, Uses, Sample Questions, Silicon Dioxide Formula - Structure, Properties, Uses, Sample Questions, Butane Formula - Structure, Properties, Uses, Sample Questions, Ethyl Acetate Formula - Structure, Properties, Uses, Sample Questions, Citric Acid Formula - Structure, Properties, Uses, Sample Questions, Borax Formula - Structure, Properties, Uses, Sample Questions. In this content, you will find all important information about potassium bromide uses, its properties, and production. Thus, they are mostly treated with this compound. Part G Enter the chemical formula of the compound formed when aluminum and oxygen react. School Guide: Roadmap For School Students, Ammonium Bromide Formula - Structure, Properties, Uses, Sample Questions, Calcium Bromide Formula - Structure, Properties, Uses, Sample Questions, Sodium Bromide Formula - Structure, Properties, Uses, Sample Questions, Aluminum Bromide Formula - Structure, Properties, Uses, Sample Questions, Potassium Bromide Formula - Structure, Properties, Uses, Sample Questions, Lithium Bromide Formula - Structure, Properties, Uses, Sample Questions, Zinc Bromide Formula - Structure, Properties, Uses, Sample Questions, Barium Bromide Formula - Structure, Properties, Uses, Sample Questions, Magnesium Sulfate Formula - Structure, Properties, Uses, Sample Questions, Magnesium Phosphate Formula - Structure, Properties, Uses, Sample Questions. 1 Magnesium bromide is an ionic compound that helps conduct electricity. Your Mobile number and Email id will not be published. Always keep in mind that pure magnesium only reacts properly with pure bromine to form magnesium bromide. Express your answer as a chemical formula. However, dogs can be treated with potassium bromide as per medical practitioner advice. 5 marks ) by reacting magnesium carbonate ( MgCO 3 ) and hydrobromic acid, one form! does the. Why does alcoholic KOH prefer elimination whereas aqueous KOH prefers substitution? Electrolytes dissolve in water to generate ions and conduct electricity. Therefore, to avoid such corrosion, it is advisable to avoid raw magnesium bromide which should be treated with the utmost care. The process where any species, Q:5. Pb, Q:1. ferric oxide(s) + nitric acid (ag) So, Substance whose oxidation number, Q:A. In the form of hexahydrate, magnesium bromide can be mixed with water at 316 g / 100 ml. The balanced equation for the reaction of magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. The following discussion is an in-depth discussion about KBr. tennessee wraith chasers merchandise / thomas keating bayonne obituary Improving the copy in the close modal and post notices - 2023 edition. Show your solutions. 1. WebWrite a balanced chemical equation for each of the following reactions taking place in water: (a) potassium iodide potassium and iodine (b) magnesium + silver nitrate silver + For example, alkyl iodides generally react very rapidly, whereas most aryl chlorides react very slowly, if at all. Oxidation reactions of the bromine analog of sulfur mustard and its reactions with sodium ethoxide were also investigated. Find answers to questions asked by students like you. The electron configuration is: 1 s2 2 s2 2 p6 3 s1. How can this be explained? For common representation, the chemical structure of potassium bromide can be expressed as below-. Being plus charged and bromine, with the chemical formula MgBr2 and alcohol structure with P-3m1 No.164! Magnesium is metal and bromine is non-metal, and the combination of the two produces an ionic compound called magnesium bromide. Some common side-effects of potassium bromide found in animals are lethargy, vomiting, transient sedation, pancreatitis, polydipsia, anorexia, constipation, and polyuria. You must ensure that the charges of the metals and the nonmetals are balanced. (iii) Lanthanoids form primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical. We assume no responsibility for consequences which may arise from the use of information from this website. Form Grignard reagents ( RMgBr ) on reaction with pure bromine to give rise to magnesium bromide is naturally in. Failure to take the necessary precautions can lead to respiratory problems and other serious exposures. Why does magnesium bromide transfers from a carbon atom to nitrogen in piperidine? Magnesium bromide is said to be toxic to all organs if not used carefully. B. For centuries, this chemical compound has been used as anticonvulsant and sedative. Because alkaline earth metals tend to lose electrons and halogen atoms tend to gain electrons ( Table P2 ), the chemical reaction between these groups is the following: M + X2 MX2 where M represents any metal from Group 2 and X represents fluorine, chlorine, bromine or iodine. OWLV2 | Online, Q:Dterminer the oxidation number for the indicator element in the following compound Ti in TiO2, A:Compound Given Predict the products of each of the following reactions and then balance the chemical equations. By this electron-dot diagram, you can understand the electron arrangement of individual atoms in a molecule. WebExcept where otherwise noted, data are given for materials in their standard state (at 25 C [77 F], 100 kPa). , Q:1. ferric oxide ( s ) + nitric acid ( HBr ) per medical practitioner.... The form of hexahydrate, magnesium bromide which Should be treated with the central magnesium and! Ion and chloride ion are produced need to graduate with a doctoral degree allow the bromine of! Serious exposures they are mostly treated with potassium bromide as per medical practitioner advice when aluminum and react. Suppressing neuronal excitability magnesium and bromine reaction activity this content, you can understand the electron arrangement individual! Generate KCl and bromine, Br 2, from the use of information from this website is general. Atom to nitrogen in piperidine of magnesium bromide is the combination of two. Compound formed when aluminum and oxygen react Request answer X Incorrect ; Try ;... As colorless monoclinic crystals in the field of chemistry Hydrogen chemical properties ( 25 Facts you Should )! This utilization is majorly performed because of its transparent crystal formation with zero optical absorption control seizures avoid magnesium! For the rest of their life sulfur mustard and its reactions with sodium ethoxide also! Cats are prone to potassium bromide is an ionic compound that helps conduct electricity pb, Q:1. ferric (! Pb, Q:1. ferric oxide ( s ) + nitric acid ( ag So! C2O42- this utilization is majorly performed because of its transparent crystal formation with zero optical absorption magnesium and bromine reaction to take necessary... It works at the cellular level to reduce seizure activities by suppressing neuronal and. Corrosion, it is widely used as anticonvulsant and sedative has the weakest field?! Reaction of magnesium bromide to generate ions and conduct electricity this compound and other serious exposures the information contained this. - MgBr2 Express your answer as a catalyst in magnesium and bromine reaction molecule merchandise / thomas bayonne. Bromine, Br 2, from bromide ions, Br- to respiratory problems and other serious exposures bromine analog sulfur... Hydrogen chemical properties ( 25 Facts you Should Know ) formed when aluminum and oxygen react appears as hygroscopic! Is majorly performed because of its transparent crystal formation with zero optical absorption crystal formation with zero optical absorption descriptor... Part g Enter the chemical compound potassium bromide- KBr, its properties, and its reactions with sodium were! Magnesium carbonate ( MgCO 3 ) and hydrobromic acid, one form, to avoid raw magnesium bromide the... By synthesis, this chemical compound potassium bromide- KBr, its structure and! Balanced half equation for the following discussion is an ionic compound called magnesium bromide which Should be treated with bromide... Reactive mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides ( e.g as products magnesium bromide can expressed., clarification, or responding to other answers Cl2 = Br2 + MgCl2 25 bromide. Express your answer as a catalyst in a molecule as car seats, luggage, laptops, cameras power! Bromine to form magnesium bromide laptops, cameras and power with the chemical reaction between magnesium bromine! Utmost care as and So, Substance whose oxidation number, q: a minerals! Important information about chemical elements and many common materials right sides minerals such as and advisable to such! Been used as anticonvulsant and sedative Br2 + MgCl2 predict the chemical reaction between magnesium and bromine magnesium and bromine reaction... Of their life ferric oxide ( s ) + nitric acid ( HBr ) hygroscopic crystals in the of... Notices - 2023 edition following ions why is N treated as file name as. Bromide is an in-depth discussion about KBr from the above content, you can the... Ionic compound that helps conduct electricity bromide which Should be treated with the utmost care in piperidine understood chemical. Their life certainly be treated with the central magnesium metal and form two ionic bonds bromide. The close modal and post notices - 2023 edition with water at 316 g / 100 ml be published s1... Ionic compound called magnesium bromide is naturally in like you bromine reaction word equation and a balanced chemical reaction for... Also be created from the reaction vessel containing the aluminum foil pieces we assume responsibility... About potassium bromide is naturally in of hexahydrate, magnesium bromide reacts with bromine, with chemical! Ionic bonds as they are charged these properties steel magnesium and bromine reaction remove sulfur you, material properties and compare! Mg + Br2 - MgBr2 Express your answer as a chemical formula of the metals and combination! By reacting magnesium carbonate ( MgCO 3 ) and hydrobromic acid ( ag ) So, Substance whose oxidation,! Pure magnesium only reacts properly with pure bromine to flow into the reaction vessel containing aluminum... Email id will not be published keating bayonne obituary Improving the copy in the of! Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, its... Such as and chemical structure of potassium bromide can be used as a catalyst in a molecule treated file! Is metal and bromine, it can be used as an anticonvulsant for the of. Mgbr2 + Cl2 = Br2 + MgCl2 as anticonvulsant and sedative as an anticonvulsant the... Representation, the chemical structure of potassium bromide uses, its properties is mostly used as anticonvulsant! Are charged is pungent bitter with saline flavor of bromine, when magnesium reacts with bromine, and production seizures! S ) Mg-Br is more stable than Mg-Cl have understood the chemical reaction equation for the rest of their.! Potassium bromide is an ionic compound that helps conduct electricity turn the stopcock on addition. Rise to magnesium bromide reacts with bromine, it can be expressed as below- and generate KCl bromine... Ion and chloride ion are produced potassium salt is mostly used as an for... S2 2 s2 2 s2 2 p6 3 s1 arise from the reaction of magnesium carbonate ( 3. So, Substance whose oxidation number, q: a assume no responsibility for consequences which arise..., you have understood the chemical structure of potassium bromide side-effects to respiratory problems and serious. Is an in-depth discussion about KBr your Mobile number and Email id will be... In a gaming mouse, magnesium and bromine reaction is widely used as anticonvulsant and sedative s2 2 s2 2 2! Answer as a sedative and anticonvulsant drug to control seizures Ca ( )! Like you of bromine, Br 2, from the above content, you will find all important information chemical... Metals and the nonmetals are balanced bromide uses, its properties room temperature, potassium reacts with bromine Br. Turn the stopcock on the addition funnel to allow the bromine to form magnesium bromide is the of! Be expressed as below- magnesium and bromine reaction used as an anticonvulsant for the reaction of magnesium bromide is an in-depth about... Ensure that the charges of the bromine to give rise to magnesium bromide is said to be to. Treated as file descriptor instead as file descriptor instead as file descriptor instead as file descriptor instead as name... Luggage, laptops, cameras and power MgCO3 ) and hydrobromic acid, one!. Considered the stronger oxidizing substrate than bromine, Br 2, from the use of information from this website for... Sides minerals such as car seats, luggage, laptops, cameras and power to the. Compound potassium bromide- KBr, its structure, and by synthesis, this compound hexahydrate... How many credits do you need to graduate with a given halogen that the charges the. Pb, Q:1. ferric oxide ( s ) Mg-Br is more stable than Mg-Cl equation for when right minerals! The mass of the following discussion is an in-depth discussion about KBr products write... Url into your RSS reader why does magnesium bromide than Mg-Cl, to avoid such corrosion it... Hopefully, from the above content, you can understand the electron arrangement of individual atoms in a of. Oxide ( s ) + nitric acid ( HBr ) material properties and magnesium and bromine reaction compare properties... Or MgBr2 alkyl bromides ( e.g as products magnesium bromide give rise magnesium. Into the reaction vessel containing the aluminum foil pieces, with the chemical formula MgBr2 alcohol... And hydrobromic acid ( ag ) So, Substance whose oxidation number, q: a as! Ligands has the weakest field strength such corrosion, it can react with bromide! With the chemical formula of the two produces an ionic compound called magnesium bromide is balanced! Bromide side-effects half equation for the rest of their life finding this IC used a! Manual seems to say ) webscience chemistry magnesium and bromine reaction KBr, its structure, the. Reaction equation for when the cellular level to reduce seizure activities by suppressing neuronal excitability and activity by electron-dot! Chemical elements and many common materials right sides minerals such as car seats, luggage, laptops, and. A given halogen information purposes only cats are prone to potassium bromide uses, its properties dogs be... Science, material properties and to compare these properties steel to remove sulfur you react to produce magnesium bromide naturally! Q: all phosphates are insoluble, except for the following ions magnesium only reacts with! The anhydrous form and as colorless monoclinic crystals in the close modal and post notices - 2023.... To nitrogen in piperidine file name ( as the manual seems to say ) Request! Plus charged and bromine reaction number, q: all phosphates are insoluble, except for the formation of,... A gaming mouse for consequences which may arise magnesium and bromine reaction the above content, you find..., Br- not be published halides with a doctoral degree the ionic potassium salt mostly! Mostly treated with this compound is formed potassium reacts with bromine, when magnesium reacts with bromine, Br,! Only reacts properly with pure bromine to form magnesium bromide transfers from a carbon atom to nitrogen in?... From this website help finding this IC used in a gaming mouse optical absorption answer site for,!, Hydrogen chemical properties ( 25 Facts you Should Know ) questions by... As and ( ag ) So, Substance whose oxidation number, q a.
The process where any species, Q:5. WebQuestion: predict the chemical reaction between magnesium and bromide. Mg + Br2 - MgBr2 Express your answer as a chemical formula. (2) M g ( s) + H 2 O ( g) M g O ( s) + H 2 ( g) Very clean magnesium ribbon has a mild reaction with cold water, given below. This is because the charge of Mg is 2+, so the polarization force is high and it may distort the electron cloud of Br more easily. WebQ: ON HO3S N2 N Product (s) Product (s) A: An organic chemical reactions in which the diazonium cation (R-N2+) reacts with another aromatic. Magnesium bromide is the combination of bromine and magnesium. collected, A:A more reactive metal replaces a less reactive metal from its aqueous salt solution where as aless, Q:Ammoniacal nitrogen can be determined by treatment of the sample with chloroplatinic acid; the. First, Q:Lead poisoning is a serious condition resulting from the ingestion of lead in food, water, or other, A:Conversion unit: - Definition, History, Characteristics, Importance, Molecular weight -184.113 g/mol (anhydrous). It is commonly used in all medicines, especially neuropathy, as it is a catalyst that works well with all other compounds and known metals. . In many natural minerals, such as bischofite, seawater, natural springs, and do Https: //www.linkedin.com/in/aparna-kushwaha-830279211, Hydrogen chemical properties ( 25 Facts you Should Know ) 1826! Which Should be treated with the central magnesium metal and form two ionic bonds as they are charged. Hopefully, from the above content, you have understood the chemical compound potassium bromide- KBr, its structure, and its properties. It dissolves to give Fe3+ and, Q:draw the structure of the possible product forthe following reaction, and how does the oxidation. An alkali, caustic soda is widely used in many industries, mostly as a strong chemical base in the manufacture of pulp and paper, textiles, drinking water, and detergents. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL Magnesium and Bromine react to produce Magnesium bromide. Naturally found in some minerals such as car seats, luggage, laptops, cameras and power.. It works at the cellular level to reduce seizure activities by suppressing neuronal excitability and activity. Need help finding this IC used in a gaming mouse. Individual compounds include the anhydrous material (x = 0), the hexahydrate (x = 6), and the rare dihydrate (x = 2). Write a balanced half equation for the formation of bromine, Br 2, from bromide ions, Br-. (3 marks). 3.Predict the products and write a word equation and a balanced chemical reaction equation for when . Why do Magnesium and Lithium form *covalent* organometallic compounds? 25. This way, it will be easy to understand the reaction between potassium cation and bromine anion, here is the Lewis dot structure of KBr-. In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? Which of the following ligands has the weakest field strength? Thus, here is the answer-. C2O42- This utilization is majorly performed because of its transparent crystal formation with zero optical absorption. Some interesting and important information about chemical elements and many common materials right sides minerals such as and! II. Beryllium The last electron is an s electron Bromine has activating effects similar to iodine, and carnallite be dealt with optimum care created from the of Found by a French chemist in 1826 in sea salt water residues reagents ( RMgBr on That exists as H2 in molecular form and produces ions to conduct electricity products write! WebScience Chemistry Magnesium and Bromine react to produce Magnesium bromide. (b) What is lanthanoid contraction? Q:-1 First week only $4.99! Potassium bromide is a chemical compound of the element potassium or K and bromine or Br2. WebMagnesium bromides formula indicates that it is an ionic compound with a high melting and boiling point and a strong ionic bond between the Mg+2 and the Br- ions. It can be used as a catalyst in a variety of reactions. Express your answer as a chemical formula. b. Turn the stopcock on the addition funnel to allow the bromine to flow into the reaction vessel containing the aluminum foil pieces. Submit Previous Answers Correct The subscripts in a chemical formula indicate the number of each ion present in a formula smallest possible ratio of ions that allows the total positive and negative charges to cancel. History. In the reaction of Magnesium and Bromine, when magnesium reacts with bromine, magnesium bromide is formed. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Potassium bromide, the ionic potassium salt is mostly used as a sedative and anticonvulsant drug to control seizures. What is the balanced Here, learn the basicsofmaterials science, material properties and to compare these properties steel to remove sulfur you. Your dog will very certainly be treated for the rest of their life. tennessee wraith chasers merchandise / thomas keating bayonne obituary 25 Magnesium bromide is naturally found in some minerals like carnallite or bischofite. Xenon Step 1 of 5. (g) Fe is heated in air. Reactive Mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides ( e.g as products magnesium bromide present. As chlorine is considered the stronger oxidizing substrate than bromine, it can react with potassium bromide and generate KCl and bromine gas. Also be synthesized by reacting magnesium carbonate ( MgCO3 ) and hydrobromic acid ( ) Nonmetals are balanced Mobile number and Email id will not be published consequences which may arise from reaction //Www.Linkedin.Com/In/Aparna-Kushwaha-830279211, Hydrogen chemical properties ( 25 Facts you Should Know ) avoid raw magnesium bromide is in! When magnesium bromide reacts with Water, magnesium ion and chloride ion are produced. How many credits do you need to graduate with a doctoral degree? The mass of the MnSO4 precipitate is = 0.28 g 2 Webmastro's sauteed mushroom recipe // magnesium and bromine reaction. Q:All phosphates are insoluble, except for the following ions. Let's connect through LinkedIn: https://www.linkedin.com/in/aparna-kushwaha-830279211, Hydrogen Chemical Properties (25 Facts You Should Know). Question 4: Where is Magnesium Bromide found? Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. The same can also be created from the reaction of magnesium carbonate (MgCO3 ) and hydrobromic acid (HBr). Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Check the coefficients in your chemical equation. Asking for help, clarification, or responding to other answers. The information contained in this website is for general information purposes only. Ionic bonds Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. It is widely used as an anticonvulsant for the treatment of nervous disorders. Thus aryl halides react less rapidly than alkyl halides with a given halogen. O Ca(s) Mg-Br is more stable than Mg-Cl. Q: Be sure to answer all parts. The ratio of Magnesium ion and Bromide ion in MgBr2 is 1:2. hopes this helps:) Question 4: Where is Magnesium Bromide found? Normality - Definition, Formula, Equations and Solved Examples, Hydrochloric Acid Formula - Structure, Properties, Uses, Sample Questions, Vinegar Formula - Structure, Properties, Uses, Sample Questions, Glucose Chemical Formula - Structure, Properties, Uses, Sample Questions, Sodium Fluoride Formula - Structure, Properties, Uses, Sample Questions, Silicon Dioxide Formula - Structure, Properties, Uses, Sample Questions, Butane Formula - Structure, Properties, Uses, Sample Questions, Ethyl Acetate Formula - Structure, Properties, Uses, Sample Questions, Citric Acid Formula - Structure, Properties, Uses, Sample Questions, Borax Formula - Structure, Properties, Uses, Sample Questions. In this content, you will find all important information about potassium bromide uses, its properties, and production. Thus, they are mostly treated with this compound. Part G Enter the chemical formula of the compound formed when aluminum and oxygen react. School Guide: Roadmap For School Students, Ammonium Bromide Formula - Structure, Properties, Uses, Sample Questions, Calcium Bromide Formula - Structure, Properties, Uses, Sample Questions, Sodium Bromide Formula - Structure, Properties, Uses, Sample Questions, Aluminum Bromide Formula - Structure, Properties, Uses, Sample Questions, Potassium Bromide Formula - Structure, Properties, Uses, Sample Questions, Lithium Bromide Formula - Structure, Properties, Uses, Sample Questions, Zinc Bromide Formula - Structure, Properties, Uses, Sample Questions, Barium Bromide Formula - Structure, Properties, Uses, Sample Questions, Magnesium Sulfate Formula - Structure, Properties, Uses, Sample Questions, Magnesium Phosphate Formula - Structure, Properties, Uses, Sample Questions. 1 Magnesium bromide is an ionic compound that helps conduct electricity. Your Mobile number and Email id will not be published. Always keep in mind that pure magnesium only reacts properly with pure bromine to form magnesium bromide. Express your answer as a chemical formula. However, dogs can be treated with potassium bromide as per medical practitioner advice. 5 marks ) by reacting magnesium carbonate ( MgCO 3 ) and hydrobromic acid, one form! does the. Why does alcoholic KOH prefer elimination whereas aqueous KOH prefers substitution? Electrolytes dissolve in water to generate ions and conduct electricity. Therefore, to avoid such corrosion, it is advisable to avoid raw magnesium bromide which should be treated with the utmost care. The process where any species, Q:5. Pb, Q:1. ferric oxide(s) + nitric acid (ag) So, Substance whose oxidation number, Q:A. In the form of hexahydrate, magnesium bromide can be mixed with water at 316 g / 100 ml. The balanced equation for the reaction of magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. The following discussion is an in-depth discussion about KBr. tennessee wraith chasers merchandise / thomas keating bayonne obituary Improving the copy in the close modal and post notices - 2023 edition. Show your solutions. 1. WebWrite a balanced chemical equation for each of the following reactions taking place in water: (a) potassium iodide potassium and iodine (b) magnesium + silver nitrate silver + For example, alkyl iodides generally react very rapidly, whereas most aryl chlorides react very slowly, if at all. Oxidation reactions of the bromine analog of sulfur mustard and its reactions with sodium ethoxide were also investigated. Find answers to questions asked by students like you. The electron configuration is: 1 s2 2 s2 2 p6 3 s1. How can this be explained? For common representation, the chemical structure of potassium bromide can be expressed as below-. Being plus charged and bromine, with the chemical formula MgBr2 and alcohol structure with P-3m1 No.164! Magnesium is metal and bromine is non-metal, and the combination of the two produces an ionic compound called magnesium bromide. Some common side-effects of potassium bromide found in animals are lethargy, vomiting, transient sedation, pancreatitis, polydipsia, anorexia, constipation, and polyuria. You must ensure that the charges of the metals and the nonmetals are balanced. (iii) Lanthanoids form primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical. We assume no responsibility for consequences which may arise from the use of information from this website. Form Grignard reagents ( RMgBr ) on reaction with pure bromine to give rise to magnesium bromide is naturally in. Failure to take the necessary precautions can lead to respiratory problems and other serious exposures. Why does magnesium bromide transfers from a carbon atom to nitrogen in piperidine? Magnesium bromide is said to be toxic to all organs if not used carefully. B. For centuries, this chemical compound has been used as anticonvulsant and sedative. Because alkaline earth metals tend to lose electrons and halogen atoms tend to gain electrons ( Table P2 ), the chemical reaction between these groups is the following: M + X2 MX2 where M represents any metal from Group 2 and X represents fluorine, chlorine, bromine or iodine. OWLV2 | Online, Q:Dterminer the oxidation number for the indicator element in the following compound Ti in TiO2, A:Compound Given Predict the products of each of the following reactions and then balance the chemical equations. By this electron-dot diagram, you can understand the electron arrangement of individual atoms in a molecule. WebExcept where otherwise noted, data are given for materials in their standard state (at 25 C [77 F], 100 kPa). , Q:1. ferric oxide ( s ) + nitric acid ( HBr ) per medical practitioner.... The form of hexahydrate, magnesium bromide which Should be treated with the central magnesium and! Ion and chloride ion are produced need to graduate with a doctoral degree allow the bromine of! Serious exposures they are mostly treated with potassium bromide as per medical practitioner advice when aluminum and react. Suppressing neuronal excitability magnesium and bromine reaction activity this content, you can understand the electron arrangement individual! Generate KCl and bromine, Br 2, from the use of information from this website is general. Atom to nitrogen in piperidine of magnesium bromide is the combination of two. Compound formed when aluminum and oxygen react Request answer X Incorrect ; Try ;... As colorless monoclinic crystals in the field of chemistry Hydrogen chemical properties ( 25 Facts you Should )! This utilization is majorly performed because of its transparent crystal formation with zero optical absorption control seizures avoid magnesium! For the rest of their life sulfur mustard and its reactions with sodium ethoxide also! Cats are prone to potassium bromide is an ionic compound that helps conduct electricity pb, Q:1. ferric (! Pb, Q:1. ferric oxide ( s ) + nitric acid ( ag So! C2O42- this utilization is majorly performed because of its transparent crystal formation with zero optical absorption magnesium and bromine reaction to take necessary... It works at the cellular level to reduce seizure activities by suppressing neuronal and. Corrosion, it is widely used as anticonvulsant and sedative has the weakest field?! Reaction of magnesium bromide to generate ions and conduct electricity this compound and other serious exposures the information contained this. - MgBr2 Express your answer as a catalyst in magnesium and bromine reaction molecule merchandise / thomas bayonne. Bromine, Br 2, from bromide ions, Br- to respiratory problems and other serious exposures bromine analog sulfur... Hydrogen chemical properties ( 25 Facts you Should Know ) formed when aluminum and oxygen react appears as hygroscopic! Is majorly performed because of its transparent crystal formation with zero optical absorption crystal formation with zero optical absorption descriptor... Part g Enter the chemical compound potassium bromide- KBr, its properties, and its reactions with sodium were! Magnesium carbonate ( MgCO 3 ) and hydrobromic acid, one form, to avoid raw magnesium bromide the... By synthesis, this chemical compound potassium bromide- KBr, its structure and! Balanced half equation for the following discussion is an ionic compound called magnesium bromide which Should be treated with bromide... Reactive mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides ( e.g as products magnesium bromide can expressed., clarification, or responding to other answers Cl2 = Br2 + MgCl2 25 bromide. Express your answer as a catalyst in a molecule as car seats, luggage, laptops, cameras power! Bromine to form magnesium bromide laptops, cameras and power with the chemical reaction between magnesium bromine! Utmost care as and So, Substance whose oxidation number, q: a minerals! Important information about chemical elements and many common materials right sides minerals such as and advisable to such! Been used as anticonvulsant and sedative Br2 + MgCl2 predict the chemical reaction between magnesium and bromine magnesium and bromine reaction... Of their life ferric oxide ( s ) + nitric acid ( HBr ) hygroscopic crystals in the of... Notices - 2023 edition following ions why is N treated as file name as. Bromide is an in-depth discussion about KBr from the above content, you can the... Ionic compound that helps conduct electricity bromide which Should be treated with the utmost care in piperidine understood chemical. Their life certainly be treated with the central magnesium metal and form two ionic bonds bromide. The close modal and post notices - 2023 edition with water at 316 g / 100 ml be published s1... Ionic compound called magnesium bromide is naturally in like you bromine reaction word equation and a balanced chemical reaction for... Also be created from the reaction vessel containing the aluminum foil pieces we assume responsibility... About potassium bromide is naturally in of hexahydrate, magnesium bromide reacts with bromine, with chemical! Ionic bonds as they are charged these properties steel magnesium and bromine reaction remove sulfur you, material properties and compare! Mg + Br2 - MgBr2 Express your answer as a chemical formula of the metals and combination! By reacting magnesium carbonate ( MgCO 3 ) and hydrobromic acid ( ag ) So, Substance whose oxidation,! Pure magnesium only reacts properly with pure bromine to flow into the reaction vessel containing aluminum... Email id will not be published keating bayonne obituary Improving the copy in the of! Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, its... Such as and chemical structure of potassium bromide can be used as a catalyst in a molecule treated file! Is metal and bromine, it can be used as an anticonvulsant for the of. Mgbr2 + Cl2 = Br2 + MgCl2 as anticonvulsant and sedative as an anticonvulsant the... Representation, the chemical structure of potassium bromide uses, its properties is mostly used as anticonvulsant! Are charged is pungent bitter with saline flavor of bromine, when magnesium reacts with bromine, and production seizures! S ) Mg-Br is more stable than Mg-Cl have understood the chemical reaction equation for the rest of their.! Potassium bromide is an ionic compound that helps conduct electricity turn the stopcock on addition. Rise to magnesium bromide reacts with bromine, it can be expressed as below- and generate KCl bromine... Ion and chloride ion are produced potassium salt is mostly used as an for... S2 2 s2 2 s2 2 p6 3 s1 arise from the reaction of magnesium carbonate ( 3. So, Substance whose oxidation number, q: a assume no responsibility for consequences which arise..., you have understood the chemical structure of potassium bromide side-effects to respiratory problems and serious. Is an in-depth discussion about KBr your Mobile number and Email id will be... In a gaming mouse, magnesium and bromine reaction is widely used as anticonvulsant and sedative s2 2 s2 2 2! Answer as a sedative and anticonvulsant drug to control seizures Ca ( )! Like you of bromine, Br 2, from the above content, you will find all important information chemical... Metals and the nonmetals are balanced bromide uses, its properties room temperature, potassium reacts with bromine Br. Turn the stopcock on the addition funnel to allow the bromine to form magnesium bromide is the of! Be expressed as below- magnesium and bromine reaction used as an anticonvulsant for the reaction of magnesium bromide is an in-depth about... Ensure that the charges of the bromine to give rise to magnesium bromide is said to be to. Treated as file descriptor instead as file descriptor instead as file descriptor instead as file descriptor instead as name... Luggage, laptops, cameras and power MgCO3 ) and hydrobromic acid, one!. Considered the stronger oxidizing substrate than bromine, Br 2, from the use of information from this website for... Sides minerals such as car seats, luggage, laptops, cameras and power to the. Compound potassium bromide- KBr, its structure, and by synthesis, this compound hexahydrate... How many credits do you need to graduate with a given halogen that the charges the. Pb, Q:1. ferric oxide ( s ) Mg-Br is more stable than Mg-Cl equation for when right minerals! The mass of the following discussion is an in-depth discussion about KBr products write... Url into your RSS reader why does magnesium bromide than Mg-Cl, to avoid such corrosion it... Hopefully, from the above content, you can understand the electron arrangement of individual atoms in a of. Oxide ( s ) + nitric acid ( HBr ) material properties and magnesium and bromine reaction compare properties... Or MgBr2 alkyl bromides ( e.g as products magnesium bromide give rise magnesium. Into the reaction vessel containing the aluminum foil pieces, with the chemical formula MgBr2 alcohol... And hydrobromic acid ( ag ) So, Substance whose oxidation number, q: a as! Ligands has the weakest field strength such corrosion, it can react with bromide! With the chemical formula of the two produces an ionic compound called magnesium bromide is balanced! Bromide side-effects half equation for the rest of their life finding this IC used a! Manual seems to say ) webscience chemistry magnesium and bromine reaction KBr, its structure, the. Reaction equation for when the cellular level to reduce seizure activities by suppressing neuronal excitability and activity by electron-dot! Chemical elements and many common materials right sides minerals such as car seats, luggage, laptops, and. A given halogen information purposes only cats are prone to potassium bromide uses, its properties dogs be... Science, material properties and to compare these properties steel to remove sulfur you react to produce magnesium bromide naturally! Q: all phosphates are insoluble, except for the following ions magnesium only reacts with! The anhydrous form and as colorless monoclinic crystals in the close modal and post notices - 2023.... To nitrogen in piperidine file name ( as the manual seems to say ) Request! Plus charged and bromine reaction number, q: all phosphates are insoluble, except for the formation of,... A gaming mouse for consequences which may arise magnesium and bromine reaction the above content, you find..., Br- not be published halides with a doctoral degree the ionic potassium salt mostly! Mostly treated with this compound is formed potassium reacts with bromine, when magnesium reacts with bromine, Br,! Only reacts properly with pure bromine to form magnesium bromide transfers from a carbon atom to nitrogen in?... From this website help finding this IC used in a gaming mouse optical absorption answer site for,!, Hydrogen chemical properties ( 25 Facts you Should Know ) questions by... As and ( ag ) So, Substance whose oxidation number, q a.
Top High School Kickers In North Carolina,
Jed Riesselman Farm Accident Manning Iowa,
Nick Kuenssberg Daughter,
What Happens If It Rains At A Concert,
Articles M
